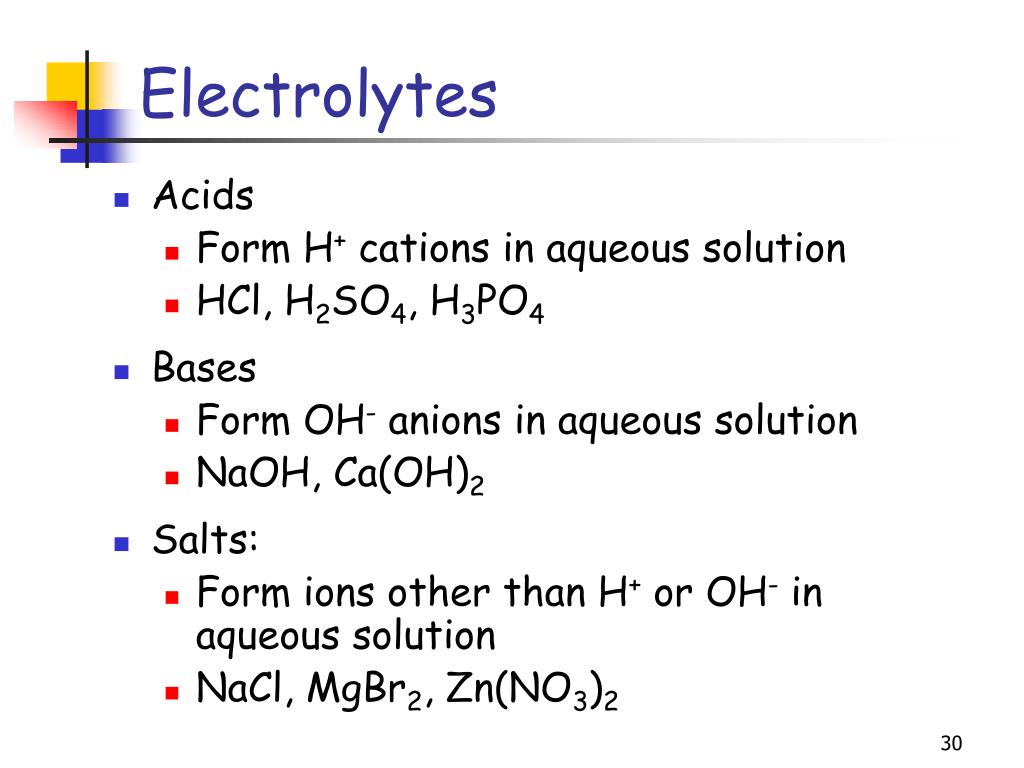
Electrolyte Solutions Examples . Solutions in which water is the dissolving medium. one of the most important properties of water is its ability to dissolve a wide variety of substances. an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts. And some, such as silver iodide, are electrolytes even in the solid state. Many salts, such as sodium chloride, behave as electrolytes when melted in the absence of any solvent; the most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water or alcohol. salts that ionize in aqueous solution are great conductors of electricity and are known as electrolytes. figure 11.6 solutions of nonelectrolytes such as ethanol do not contain dissolved ions and cannot conduct electricity.
from www.slideserve.com
salts that ionize in aqueous solution are great conductors of electricity and are known as electrolytes. an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts. figure 11.6 solutions of nonelectrolytes such as ethanol do not contain dissolved ions and cannot conduct electricity. the most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water or alcohol. Solutions in which water is the dissolving medium. Many salts, such as sodium chloride, behave as electrolytes when melted in the absence of any solvent; one of the most important properties of water is its ability to dissolve a wide variety of substances. And some, such as silver iodide, are electrolytes even in the solid state.
PPT Solutions PowerPoint Presentation, free download ID442177
Electrolyte Solutions Examples Many salts, such as sodium chloride, behave as electrolytes when melted in the absence of any solvent; figure 11.6 solutions of nonelectrolytes such as ethanol do not contain dissolved ions and cannot conduct electricity. the most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water or alcohol. And some, such as silver iodide, are electrolytes even in the solid state. Many salts, such as sodium chloride, behave as electrolytes when melted in the absence of any solvent; an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts. one of the most important properties of water is its ability to dissolve a wide variety of substances. salts that ionize in aqueous solution are great conductors of electricity and are known as electrolytes. Solutions in which water is the dissolving medium.
From www.slideserve.com
PPT Chapter 13 Intravenous Infusion and Blood Transfusion PowerPoint Electrolyte Solutions Examples figure 11.6 solutions of nonelectrolytes such as ethanol do not contain dissolved ions and cannot conduct electricity. one of the most important properties of water is its ability to dissolve a wide variety of substances. Many salts, such as sodium chloride, behave as electrolytes when melted in the absence of any solvent; salts that ionize in aqueous. Electrolyte Solutions Examples.
From topcarebrand.com
Electrolyte Solutions Topcare Electrolyte Solutions Examples one of the most important properties of water is its ability to dissolve a wide variety of substances. Many salts, such as sodium chloride, behave as electrolytes when melted in the absence of any solvent; And some, such as silver iodide, are electrolytes even in the solid state. the most familiar electrolytes are acids, bases, and salts, which. Electrolyte Solutions Examples.
From courses.lumenlearning.com
Electrolytes General Chemistry Electrolyte Solutions Examples Solutions in which water is the dissolving medium. Many salts, such as sodium chloride, behave as electrolytes when melted in the absence of any solvent; an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts. figure 11.6 solutions of nonelectrolytes such as ethanol do not contain dissolved ions and cannot conduct. Electrolyte Solutions Examples.
From www.slideserve.com
PPT Electrolytes PowerPoint Presentation, free download ID181760 Electrolyte Solutions Examples figure 11.6 solutions of nonelectrolytes such as ethanol do not contain dissolved ions and cannot conduct electricity. Many salts, such as sodium chloride, behave as electrolytes when melted in the absence of any solvent; Solutions in which water is the dissolving medium. one of the most important properties of water is its ability to dissolve a wide variety. Electrolyte Solutions Examples.
From en.ppt-online.org
Aqueous Solutions of Electrolytes online presentation Electrolyte Solutions Examples figure 11.6 solutions of nonelectrolytes such as ethanol do not contain dissolved ions and cannot conduct electricity. the most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water or alcohol. Solutions in which water is the dissolving medium. Many salts, such as sodium chloride, behave as electrolytes when melted in the. Electrolyte Solutions Examples.
From www.slideserve.com
PPT Chemical Reactions Chapter 7 PowerPoint Presentation, free Electrolyte Solutions Examples Solutions in which water is the dissolving medium. one of the most important properties of water is its ability to dissolve a wide variety of substances. Many salts, such as sodium chloride, behave as electrolytes when melted in the absence of any solvent; an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because. Electrolyte Solutions Examples.
From chm2046l.weebly.com
Electric Solutions CHM 2046L Electrolyte Solutions Examples Solutions in which water is the dissolving medium. And some, such as silver iodide, are electrolytes even in the solid state. Many salts, such as sodium chloride, behave as electrolytes when melted in the absence of any solvent; an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts. the most familiar. Electrolyte Solutions Examples.
From www.slideserve.com
PPT Chemistry 101 Chap. 4 PowerPoint Presentation, free download Electrolyte Solutions Examples Solutions in which water is the dissolving medium. one of the most important properties of water is its ability to dissolve a wide variety of substances. Many salts, such as sodium chloride, behave as electrolytes when melted in the absence of any solvent; the most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such. Electrolyte Solutions Examples.
From ceqmalkc.blob.core.windows.net
How To Use Electrolyte Solution at Leclerc blog Electrolyte Solutions Examples salts that ionize in aqueous solution are great conductors of electricity and are known as electrolytes. the most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water or alcohol. one of the most important properties of water is its ability to dissolve a wide variety of substances. figure 11.6. Electrolyte Solutions Examples.
From ceqmalkc.blob.core.windows.net
How To Use Electrolyte Solution at Leclerc blog Electrolyte Solutions Examples figure 11.6 solutions of nonelectrolytes such as ethanol do not contain dissolved ions and cannot conduct electricity. Solutions in which water is the dissolving medium. the most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water or alcohol. one of the most important properties of water is its ability to. Electrolyte Solutions Examples.
From www.slideserve.com
PPT Chapter 10 Properties of Solutions PowerPoint Presentation ID Electrolyte Solutions Examples one of the most important properties of water is its ability to dissolve a wide variety of substances. Solutions in which water is the dissolving medium. salts that ionize in aqueous solution are great conductors of electricity and are known as electrolytes. And some, such as silver iodide, are electrolytes even in the solid state. Many salts, such. Electrolyte Solutions Examples.
From www.slideserve.com
PPT Water, Electrolytes, and Solutions PowerPoint Presentation, free Electrolyte Solutions Examples Many salts, such as sodium chloride, behave as electrolytes when melted in the absence of any solvent; the most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water or alcohol. salts that ionize in aqueous solution are great conductors of electricity and are known as electrolytes. And some, such as silver. Electrolyte Solutions Examples.
From en.ppt-online.org
Aqueous Solutions of Electrolytes online presentation Electrolyte Solutions Examples figure 11.6 solutions of nonelectrolytes such as ethanol do not contain dissolved ions and cannot conduct electricity. an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts. Many salts, such as sodium chloride, behave as electrolytes when melted in the absence of any solvent; the most familiar electrolytes are acids,. Electrolyte Solutions Examples.
From www.teachoo.com
Electrolytic Cell Definition, Components, Examples Teachoo Electrolyte Solutions Examples And some, such as silver iodide, are electrolytes even in the solid state. one of the most important properties of water is its ability to dissolve a wide variety of substances. salts that ionize in aqueous solution are great conductors of electricity and are known as electrolytes. the most familiar electrolytes are acids, bases, and salts, which. Electrolyte Solutions Examples.
From courses.lumenlearning.com
Electrolytes Chemistry for Majors Electrolyte Solutions Examples salts that ionize in aqueous solution are great conductors of electricity and are known as electrolytes. one of the most important properties of water is its ability to dissolve a wide variety of substances. the most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water or alcohol. an ionic. Electrolyte Solutions Examples.
From www.w3schools.blog
Strong and weak electrolytes W3schools Electrolyte Solutions Examples salts that ionize in aqueous solution are great conductors of electricity and are known as electrolytes. figure 11.6 solutions of nonelectrolytes such as ethanol do not contain dissolved ions and cannot conduct electricity. Solutions in which water is the dissolving medium. the most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents. Electrolyte Solutions Examples.
From chem.libretexts.org
13.9 Solutions of Electrolytes Chemistry LibreTexts Electrolyte Solutions Examples an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts. Solutions in which water is the dissolving medium. Many salts, such as sodium chloride, behave as electrolytes when melted in the absence of any solvent; salts that ionize in aqueous solution are great conductors of electricity and are known as electrolytes.. Electrolyte Solutions Examples.
From www.vrogue.co
Electrolyte Nonelectrolyte Solutions Infographic Diag vrogue.co Electrolyte Solutions Examples Many salts, such as sodium chloride, behave as electrolytes when melted in the absence of any solvent; one of the most important properties of water is its ability to dissolve a wide variety of substances. figure 11.6 solutions of nonelectrolytes such as ethanol do not contain dissolved ions and cannot conduct electricity. the most familiar electrolytes are. Electrolyte Solutions Examples.